HOW IT WORKS
fILL OUT THE FORM
SUBMIT A CLAIM
get a free case review
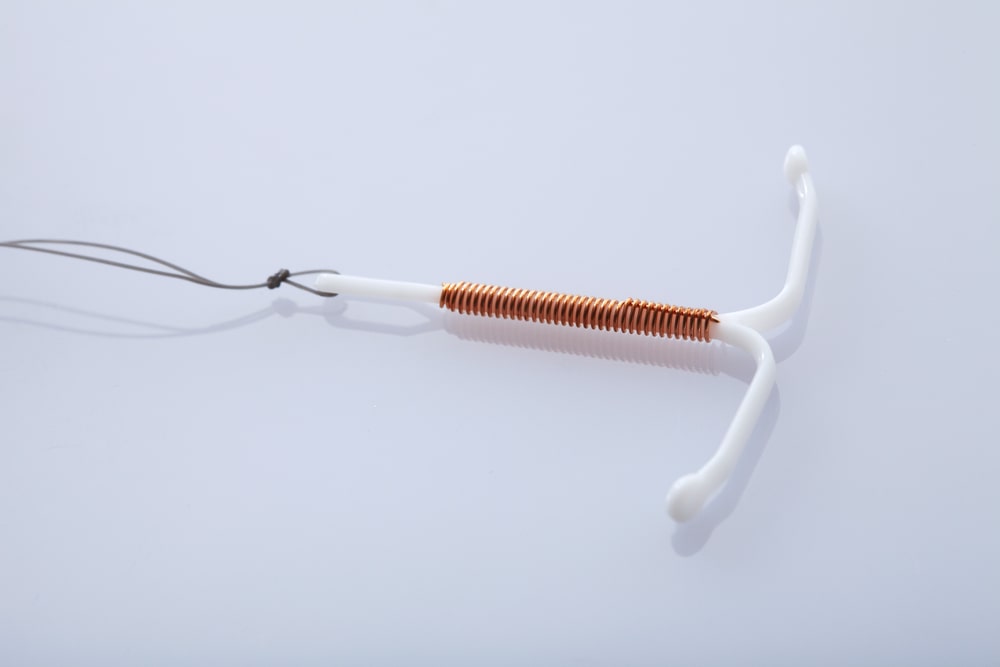
Legal Claims Are Being Filed For Compensation
those who have had the paragard® device implanted may be entitled to compensation. when the device is implanted, it may cause uterus perforation or scarring. additionally, many patients have suffered from breakages or fractures of the device during the removal process.
these circumstances may require that a patient undergo additional operations for the safe removal of the device and to fix any damage it may have caused. those who have been impacted by the paragard® device may be entitled to compensation.
Please seek the advice of a medical professional before making health care decisions. This advertisement is not associated with Paragard®or any government agency.
ATTORNEY ADVERTISING. This Website is not intended to provide medical advice. Consult your doctor or physician before starting or stopping any medication.
Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. are not an indication of future results. Every case is evaluated on its own facts and circumstances. Valuation depends on facts, injuries, jurisdiction, venue, witnesses, parties, and testimony, among other factors. No representation is made that the quality of legal services to be performed is greater than the quality of legal services performed by other lawyers. Online Justice Group does not itself provide legal services. Cases will be referred to third party attorneys and law firms. not rely on this advertisement in making any medical decision. Please call your physician before making any medical decision, including altering your use of any drug. Court costs and case expenses may be the responsibility of the client. Not available in all states. This advertisement is not intended as a testimonial, endorsement or dramatization, and does not constitute a guarantee, warranty, or prediction regarding the outcome of your legal matter, either express or implied. Anyone considering a lawyer should independently investigate the lawyers' credentials and ability, and not rely upon advertisements or self-proclaimed expertise.
Privacy Policy | Terms and Conditions | CCPA Privacy Notice | Do Not Sell My Info
